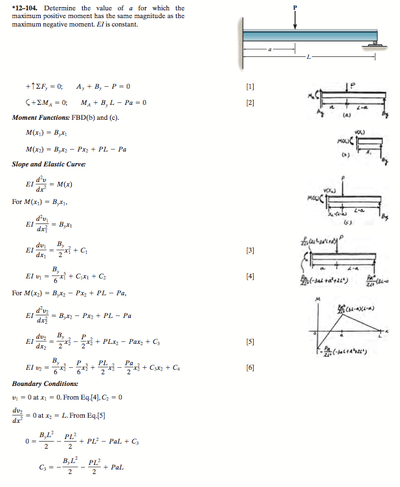
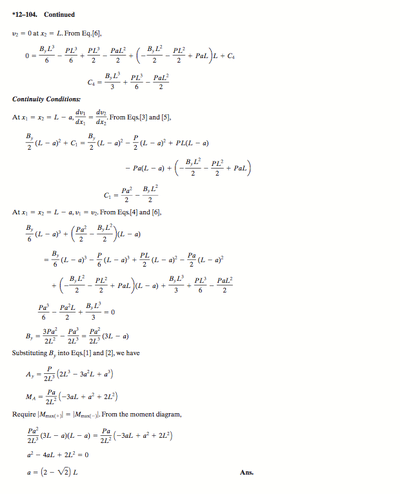Density Worksheet
Name__________________________________
Density is the ratio of the mass of the substance to the volume of the substance.
Density has units of g/ cm3 or g/ml for liquids and solids, and g/L for gases.
1. The mass of an unknown type of metal is 51 g, and its volume is 4.5 cm3. What is the density of this metal?
2. What volume would a 0.871 gram sample of air occupy if the density of air is 1.29 g/L?
3. A gold-colored ring has a mass of 18.9 grams and a volume of 1.12 ml. Is the ring pure gold? (The density of gold is 19.3 g/ml.)
4. Pumice is volcanic rock that contains many trapped air bubbles. A 225 gram sample occupied 236.6 ml. What is the density of pumice? (Answer is 0.951 g/ml)
Will pumice float on water? (The density of water is 1.0 g/ml.)
5. A cup of sugar has a volume of 237 ml. What is the mass of the cup of sugar if the density is 1.59 g/ml? (Ans. is 377 grams)
6. Which has the greater mass, 1 litre of water or 1 litre of gasoline? The density of water is 1.00 g/ml and that of gasoline is approximately 0.68 g/ml
7. A crumpet recipe calls for 175 grams of flour. According to Mr. Lecuyer’s data, the density of flour is 0.620 g/ml. How many ml of flour are needed for this recipe? (Ans. is 282 ml)
8. From their density values, decide whether each of the following substances will sink or float when placed in sea water, which has a density of 1.025 g/ml.
Gasoline 0.66 g/ml Asphalt 1.2 g/ml
Mercury 13.6 g/ml Cork 0.26 g/ml
9. A sample of lead is found to have a mass of 32.6 g. A graduated cylinder contains 2.8 ml of water. After the lead sample is added to the cylinder the water level reads 5.7 ml. Calculate the density of the lead sample. (11g/mL)